Metanephrine ELISA kit
High Sensitivity – Plasma samples
Ref: BA-E-8100
 +
+Plasma metanephrine ELISA kit
DatasheetMSDSProtocol
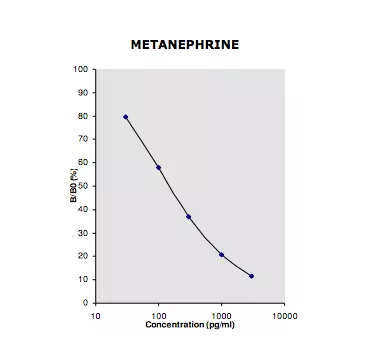
This Metanephrine ELISA kit enables the measurement of free L-metanephrine in plasma samples with high sensitivity. Intended for in vitro diagnostics & research use, this quantitative immunoassay is based on a simple and fast sample preparation protocol, and offers a limit of detection as low as 17pg/ml.
| Sample type | 500µl plasma |
| Samples/kit | 40 samples in duplicate |
| Sensitivity | 17pg/ml |
| Range | 36 – 3 600pg/ml |
| Assay time | Sample preparation and acetylation (30 min), ELISA overnight |
| Reactivity | Reacts with all species |
| Citations | Cited in 1 paper |
Product overview
| Product name | Metanephrine ELISA kit – Plasma |
| Description | In vitro diagnostics & Research enzyme immunoassay (ELISA) for the sensitive determination of free metanephrine (metadrenaline) in plasma samples |
| Labels | IVD (EU only) or RUO, CE |
| Format | 96-well plate |
| Samples | plasma |
| Sample volume | 500µL |
| Reactivity | Reacts with all species |
| Standard range | 0 / 36 – 3 600pg/ml |
| Sensitivity |
17pg/ml |
| Specificity | No significant cross-reactivity was observed with Metanephrine analogs such as Normetanephrine, 3-methoxytyramine, Adrenaline, Noradrenaline, Dopamine, VMS, HVMS, L-Dopa, L-Tyrosine, Tyramine and Acetaminophen |
| Assay time | Sample preparation and acetylation 30 min and ELISA overnight |
| Storage | Store at 2-8°C for to 6 months |
| Datasheets | Instructions for use, Material safety datasheet |
| Sample collection & storage | EDTA- or citrate-plasma sample only. Do not use haemolytic or lipemic samples. Storage: up to 6 hours at 2 – 8°C; for longer periods (up to 6 months) at – 20°C |
| Sample preparation | Sample precipitation and acetylation (30min) |
| ELISA | Metanephrine antiserum overnight incubation, revelation and read steps (1h) |
| Detailed protocol | Download instructions for use |
Product citation
- Generation of a mouse model of thyroid storm and preliminary investigation of the therapeutic effects of ghrelin
Check the article
Authors : Kyrimoto et al., BMC Endocrine Disorders
2024-08